Mixtures Can Be Separated By
Separation of Mixtures or method of separation is the process of separating or extracting different components of a mixture using some physical methods. The type of mixture and variations in the chemical characteristics of the mixture's components determine the separation method to be used. Separation of mixtures is oftentimes needed to get rid of unwanted or harmful components and to carve up the useful components from the non-useful ones. Some of the methods of the mixtures explained in detail in this article are Filtration, Paw-picking, Threshing, Winnowing, Sieving, Sedimentation and decantation, Magnetic separation, Centrifugation, Evaporation, Distillation, and many more.
Method of Separation of Mixtures
Dissimilar methods of separation are used depending on the type of mixture. Some commonly used methods for separation include:
- Sublimation
- Evaporation
- Handpicking
- Threshing
- Winnowing
- Sieving
- Distillation
- Filtration or Sedimentation
- Separating Funnel
- Magnetic Separation
Permit us understand them in more than item below.
Separation of Mixtures by Sublimation
A mixture with i sublimable volatile component and one non-sublimable component—ordinarily referred to as impurity—can be separated using the sublimation process.
Let'southward speedily understand the sublimation method for separation:
- Take the common salt and ammonium chloride combination and put information technology in a china dish to divide it.
- Next, set an upside-downwardly funnel on the communist china dish with a cotton stopper at the stop to stop volatile substances' vapors from escaping.
- This combination should be heated for a while.
- The sublime ammonium chloride vapourises, cools, and and then condenses back to solid form on the funnel's inner walls.
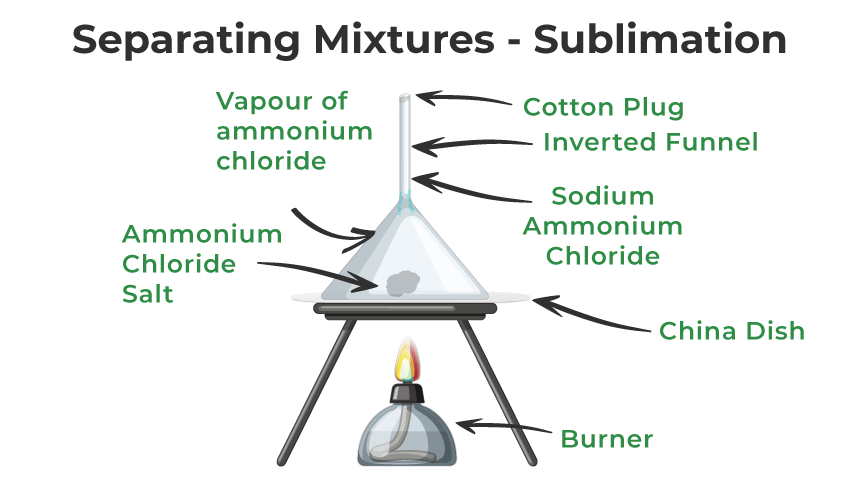
In this way, sublimation can be used to dissever the sublimable parts of a mixture from the not-sublimable parts.
Separation of Mixtures past Evaporation
Evaporation is a technique for separating a mixture, most unremarkably a solution of a solvent and a soluble solid. The solution is heated until the organic solvent evaporates, turning into a gas and mostly leaving behind the solid residue in this method.

Evaporation is a method of separating homogeneous mixtures containing ane or more dissolved salts. The method separates the liquid from the solid components. Typically, the process entails heating the mixture until no liquid remains. Unless information technology is not necessary to isolate the liquid components, the mixture should only incorporate ane liquid component before using this method. This is due to the fact that all liquid components will evaporate over time. Evaporation is an effective method for separating a soluble solid from a liquid.
Separation of Mixtures by Handpicking
Handpicking is used to carve up mixtures where one of the components is in small quantities. Handpicking is a technique used to separate undesirable substances such as pocket-sized pieces of rock from wheat, rice, and pulses.

Food grains with small pieces of stone are transported in a flat container. Hands pick upwards the stones from the grains one past 1 and throw them away. Only nutrient grains remain after all the rock fragments have been removed.
Separation of Mixtures by Threshing
When a food grain crop, such every bit wheat or paddy, reaches maturity, information technology is harvested from the field. The harvested crop is then lord's day-stale. We receive bundles of dried ingather plant stems or stalks with grains attached at the pinnacle. A thin layer of crust covers the grains attached to the stems or stalks. Each stalk is densely packed with chaff-covered grains. Grains are separated from stems and stalks, as well as chaff.
The process of threshing separates the grains from the stems or stalks. Threshing is chirapsia wheat or paddy stems to split up grains from the stems and the chaff that covers the grains.

Ingather plant stalks and stems, also every bit chaff, are soft materials, whereas grains are extremely difficult. Because the stalks and chaff are soft, they can be broken into pieces when beaten, simply the grains are unaffected. Threshing is accomplished by holding bundles of stems in ane'southward hands and hit them against a hard surface. As a result, the grains separate from the stems.
Threshing is too done with the assist of cattle. The harvested and stale ingather plants are spread on the ground in a pocket-sized expanse, and diverse cattle such as buffaloes and camels are made to walk in circles over them for an extended flow of time. The cattle'due south feet crush the stems or stalks, separating the grains from the stems. This burdensome also breaks the chaff surrounding the grains, allowing the grains to be separated from the chaff. During the threshing process, the stalks are reduced to very minor pieces known as hay, which is used as dry fodder for cattle. The husk is fabricated up of broken crust. For the threshing process, a motorized motorcar known equally a thresher is used.
Separation of Mixtures by Winnowing
When a farmer threshes wheat in his field, he gets a mixture of wheat grains and husk. Husk must be removed from wheat grains before they can be used. The husk is separated from the wheat grains by winnowing.
Winnowing is the process of separating husk from grains using the wind. Wheat grains are heavy, whereas husk is calorie-free. Winnowing is accomplished with a winnowing handbasket. The farmer stands on a higher platform than the basis and shakes his winnowing basket continuously to allow the mixture of wheat grains and husk to fall from a elevation.
Because wheat grains are heavy, they fall vertically to the basis, forming a heap of wheat grains. Because husk particles are lighter, they are carried further by the wind. Equally a event, the husk forms a separate heap abroad from the wheat grain heap. In this manner, the husk is separated from the wheat grains.
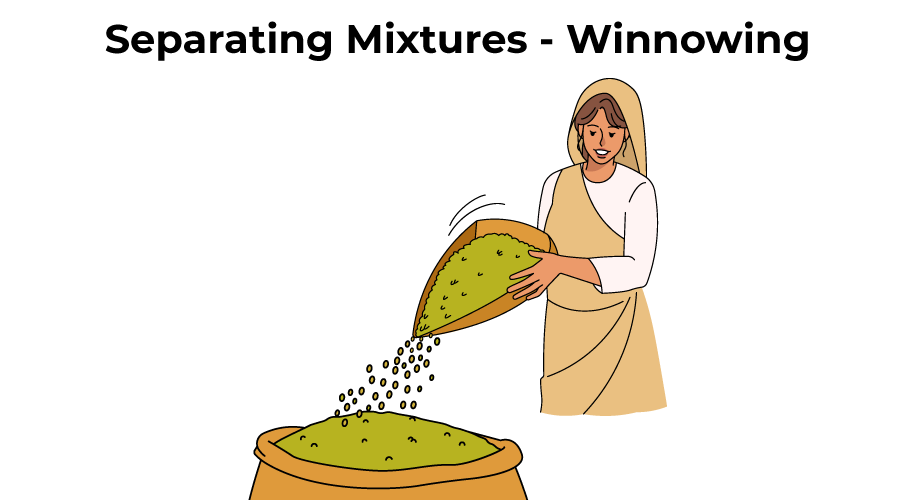
Winnowing is a technique used to remove the husk from grains such as wheat and rice. Nosotros cannot separate pocket-size stone particles from wheat using the winnowing process. This is due to the fact that stone particles are quite heavy and cannot be carried a long distance by wind.
Separation of Mixtures by Sieving
A sieve is a shallow container with minor holes at the bottom. In some cases, an atomic number 26 mesh tin also be used as a sieve. Sieving is the process of parting a mixture using a sieve. Sieving is used to separate solid mixtures that incorporate components of varying sizes.
The mixture, which contains components of varying sizes, is placed in a sieve, and the sieve is continuously moved dorsum and forth. The larger particles of the mixture cannot pass through the sieve's small holes and thus remain trapped in the sieve.
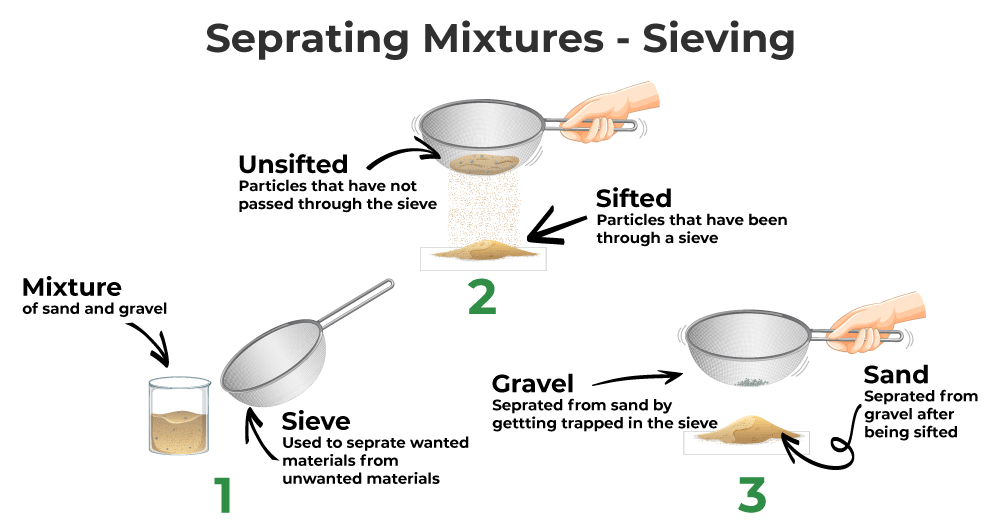
The mixture separates into 2 components: one with modest particles and 1 with larger particles. The size of the sieve'south holes is determined by the size of the particles of the material to exist separated from the mixture. Different substances are separated using sieves with varying sizes of holes.
- Wheat brought from the fields however contains impurities such as stones, stalks, and husks. Impurities are removed from wheat before grinding in a flour mill via a sieving procedure. A bag of wheat is poured through an iron mesh slanting sieve. The wheat grains pass through the Sieve, leaving backside pieces of stone, talk, and husk.
- Sieving is used to obtain fine sand. Coarse sand with larger particles and pebbles is placed on a sieve made of a large fe held in a slanting position. Fine sand particles pass through the fe mesh merely are left behind.
- Sieving is also used to separate like objects of varying sizes. Cashew basics of various sizes are separated in cashew nut factories through the sieving process.
Separation of Mixtures past Distillation
Distillation is a fast way to split up mixtures of two or more pure liquids. Distillation is a purification method that involves vaporizing the constituents of a liquid mixture, so condensing and isolating them.
A mixture is heated in elementary distillation, and the most volatile component vaporizes at the lowest temperature. The vapor condenses back into liquid after passing through a cooled tube (a condenser). The collected condensate is known as distillate.
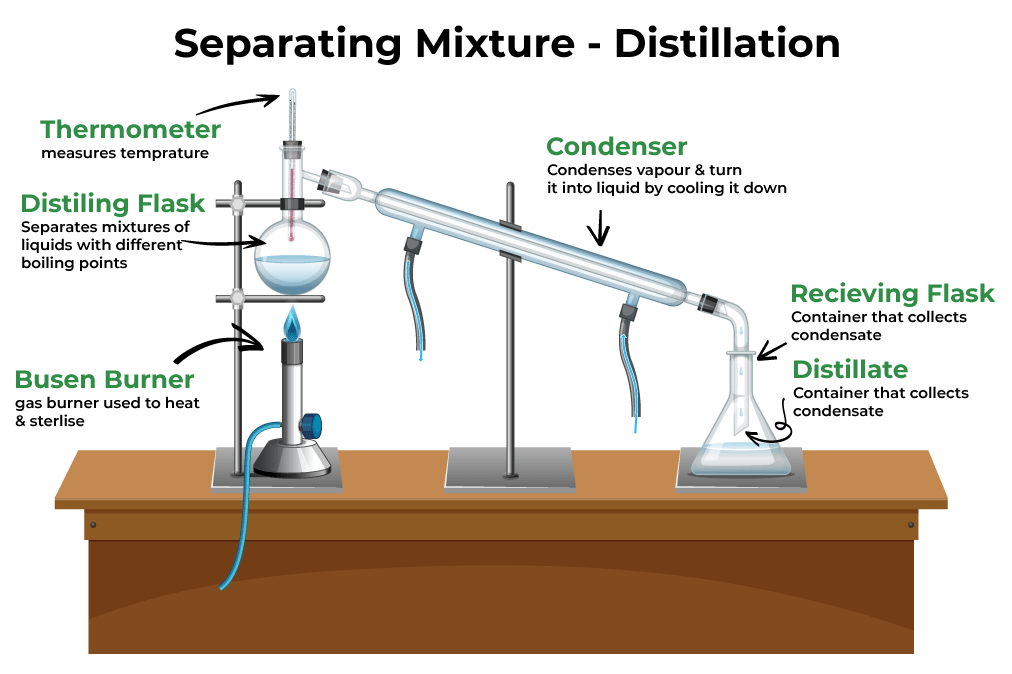
Several important pieces of equipment are depicted in the effigy above. A heat source, a test tube with a ane-pigsty stopper attached to a glass elbow, and rubber tubing are all present. The safety tubing is inserted into a collection tube filled with common cold water. Other more than complicated distillation assemblies tin can as well be used, specially to separate mixtures of pure liquids with similar boiling points.
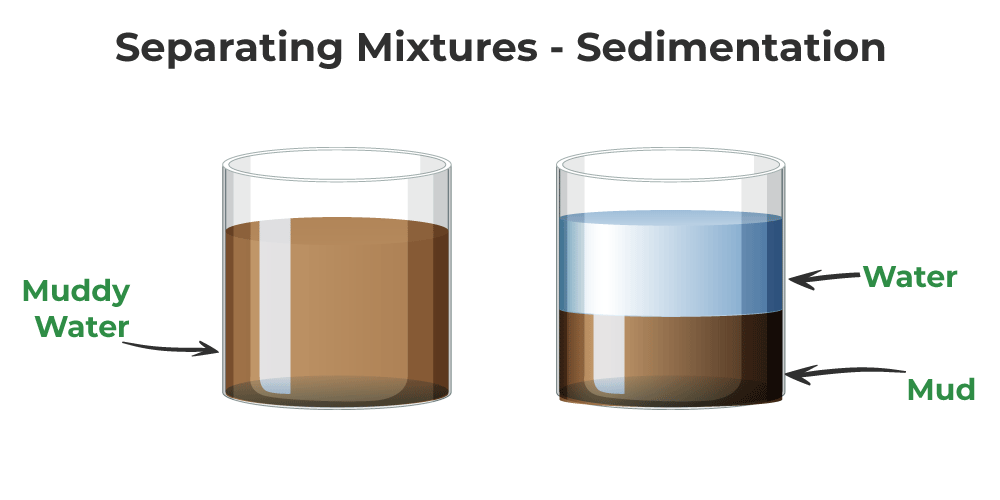
Separation of Mixtures by Filtration or Sedimentation
The most common method of separating a liquid from an insoluble solid is filtration. Consider the case of a sand-water mixture. In this case, filtration removes solid particles from the liquid. Various filtering agents, such as filtering paper or other materials, are commonly used.

Sedimentation is the process by which heavier impurities in a liquid, typically water, settle to the lesser of the container containing the mixture. It takes some time to complete the process.
Separation of Mixtures by Separating Funnel
This method is used with immiscible liquids (those that do non mix together). The machinery works past exploiting the diff density of the particles in the mixture. Using this technique, oil and water tin can be easily separated. In the process, a separating funnel is used.
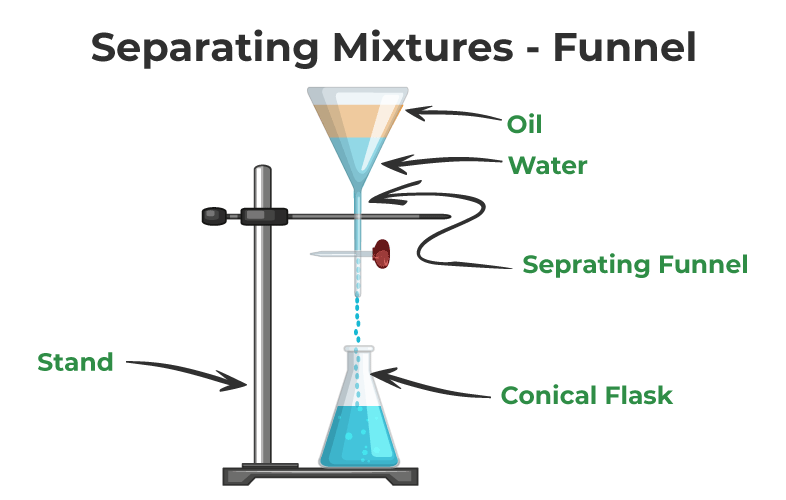
Once the liquids are in the funnel, you must expect for them to grade two layers. The denser liquid sinks to the bottom, while the other liquid rises to the surface. At the bottom, a conical flask is placed to collect the denser liquid. The valve allows yous to control when and how much liquid is allowed to pass through to the conical flask.
Separation of Mixtures by Magnetic Separation
Magnetization is a method of attracting magnetic materials. Magnetic separation is the process of separating mixtures of 2 solids, one of which has magnetic properties.

It is based on the distinction between magnetic and not-magnetic materials. Fe particles in the mixture of iron and Sulphur are attracted to the magnet and carve up from the not-magnetic substance.
FAQs on Separation of Mixtures
Question 1: What is the principle used in the Separation of Mixtures?
Reply:
A distinctive holding of one of the mixture's components is used every bit the basis for the separation of mixture principle. For case, using a magnet to separate iron bits mixed with sand is possible considering iron is drawn to the magnet.
Question 2: Explain why in that location is a need for the Separation of Mixtures.
Answer:
The separation of mixtures is needed because to split the useful from the undesirable or potentially harmful ones, nosotros must carve up the various components of a mixture.
Question 3: What is Separation of Mixture mean?
Reply:
A mixture is composed of more one pure grade of matter. Mixtures are classified as either homogeneous or heterogeneous. Homogeneous mixtures have a uniform limerick, whereas heterogeneous mixtures do not. Mixture separation refers to the separation of homogeneous and heterogeneous mixtures.
Question 4: What method will y'all apply to separate a sand and sugar mixture?
Reply:
The carbohydrate would deliquesce in water. After that, the solution could exist poured off and the remaining sand washed with a niggling more water. Separate the 2 by heating the water to carve up it from the carbohydrate. In h2o, the sugar would dissolve. The solution could then exist poured off and the remaining sand washed with a niggling more than h2o. Rut the water to split it from the saccharide, and so separate the two.
Question 5: Explain how to carve up a sand-salt mixture.
Answer:
To divide common salt and sand from a mixture, the processes of filtration and evaporation are used. Filtration tin separate sand from a sand-table salt solution mixture (salt dissolved in water). After filtration, sand remains as a residue on the filter paper. Common common salt tin can at present be obtained by boiling the filtrate. When water boils, information technology evaporates completely, leaving but table salt backside. This is referred to every bit evaporation.
Question half-dozen: What method will you employ to split a salt and sugar mixture?
Answer:
Evaporation (the procedure of converting a liquid to vapour) tin separate the sugar and common salt solution, and if the water is completely evaporated, we will get separated sugar from the mixture, whereas if the solution is dissolved in booze, we will get salt while carbohydrate volition be dissolved in booze.
Question vii: What is the name of the solvent used to separate a Sulphur and Carbon mixture?
Answer:
To separate the carbon and Sulphur mixture, a carbon disulphide solution tin can be used. Carbon is undissolved in the solution, but Sulphur dissolves. This mixture is then filtered to remove the carbon before beingness evaporated to extract the Sulphur.
Question eight: What is the method and process to separate a mixture of sodium chloride and sand?
Answer:
The filtration and distillation processes can exist used to carve up a mixture of sodium chloride and sand in water. When the solution is filtered, the sand remains on the filter newspaper while the liquid passes through. The solution is then distilled. During distillation, water evaporates, then condenses and cools to go liquid. H2o volition be collected in the chalice. Meanwhile, salt will remain in the distillation flask as a residue.
Question 9: What method will you use to separate iodine from an iodine-and-mutual-common salt mixture?
Answer:
is the almost constructive method for separating iodine from common table salt (NaCl). Considering iodine is sublimable, it volition alter from solid to vapour when slightly heated, and the iodine vapours can be collected while common table salt remains solid.
Question 10: Explicate the method to divide a mixture of iron filling and powdered carbon.
Answer:
Using magnetic separation We can split up a mixture of atomic number 26 filings and powdered carbon using a magnet. The allure of magnets by iron serves equally the foundation for the separation of atomic number 26 fillings and powdered carbon.
Related Manufactures
What are Mixtures?
Separation of Mixtures of Two or More Liquids
How to split a Mixture of Two Solids?
Mixtures Can Be Separated By,
Source: https://www.geeksforgeeks.org/separation-of-mixtures/
Posted by: ransommingenty.blogspot.com


0 Response to "Mixtures Can Be Separated By"
Post a Comment